| SpectraBase Spectrum ID |
39NkZGshRjZ |
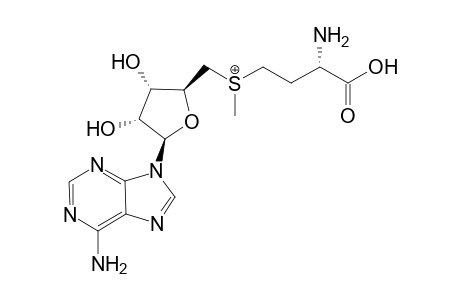
| Name |
s-(5'-Adenosyl)-L-methionine |
| Acquisition Mode |
SIMULTANEOUS |
| CAS Registry Number |
23095-97-8
2613-02-7
28378-99-6
29908-03-0
5134-37-2
86522-35-2
86866-89-9 |
| ChEBI ID |
15414 |
| Comments |
100 mM s-(5'-adenosyl)-L-methionine chloride - vendor: Sigma A7007; Solvent: D2O; Buffers, etc: 50 mM Sodium Phosphate, 500 uM NaAzide; Temperature=298 K, pH=7.4; NMR Reference: 500 uM DSS; Bruker DMX 400MHz
(Data collected by Madison Metabolomics Consortium) |
| Copyright |
Database Compilation Copyright © 2021-2024 John Wiley & Sons, Inc. All Rights Reserved. |
| Data Source |
Madison Metabolomics Consortium |
| Formula |
C15H22N6O5S |
| IUPAC Name |
(2S)-2-amino-4-[[(2S,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methyl-methyl-sulfonio]butanoate; (2S)-2-amino-4-[[(2S,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl]methyl-methyl-sulfonio]butanoate |
| InChI |
InChI=1S/C15H22N6O5S/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/p+1/t7-,8+,10+,11+,14+,27?/m0/s1 |
| InChIKey |
MEFKEPWMEQBLKI-AIRLBKTGSA-O |
| KEGG Compound ID |
C00019 |
| KEGG Pathways |
PATH: map00220 Urea cycle and metabolism of amino groups
PATH: map00271 Methionine metabolism
PATH: map00640 Propanoate metabolism |
| PubChem Compound ID |
34755 |
| SMILES |
C[S+](CCC(C(=O)[O-])N)CC1C(C(C(O1)N2C=NC3=C2N=CN=C3N)O)O |
| Source File Reference |
bmse000059 |